Crushing the Countdown Timer. One Kidney Provides >50 Years of Function
Crushing the Countdown Timer. One Kidney Provides >50 Years of Function
Is there’s a hidden countdown timer on transplanted kidneys? According to an article in USA Today, two 60-something year old women shattered that belief by surpassing the 50-year mark on their “one and only.”
Those remarkable women are Sharon Stakofsky-Davis and Denice Lombard. Both Sharon and Denice received their transplanted kidneys from their respective fathers when they were young teenagers. More than a half-century later, both women are still around, as is Denice’s father, at nearly 95—and the kidney he donated to her. Sharon’s dad died several years ago (unrelated to donation), and now the kidney he gave her is essentially dying too. But, boy what a run!
Imagine a transplanted kidney lasting for 51-years, like Sharon’s did— or for 56-years (and still going), like Denice’s. While it’s beyond noteworthy to admire these outliers, let’s advance the dialogue to help more post-transplant patients follow their lead.
We can do this by expanding living donation and preemptive transplantation opportunities, and by encouraging pharma to fill their pipelines with less harmful immunosuppressive therapies. Of equal importance, we must constantly work to reduce the number of deaths (and disqualifying illnesses) for those waiting on the list.
I believe these milestones will cause our transplant community to pause, reflect and propel forward. After all, the reality of achieving five-plus decades of post-transplant function (from one transplanted kidney) is no longer an illusion. Rarely reported, yes. But no longer impossible. Perhaps receiving “one kidney for life” has made its way a bit closer to the goal line.
USA Article Link: https://www.usatoday.com/story/news/nation/2023/09/05/kidney-transplant- organ-longevity-advances/70722404007/
 Biden Signs Bill Introducing More Competition to US’ Organ Transplant Network
Biden Signs Bill Introducing More Competition to US’ Organ Transplant Network
On September 22, 2023, President Joe Biden signed a bipartisan bill into law that overhauls our country’s organ transplant system by increasing competition among contractors—and paving the way for additional funding.
The new law will “break up the current monopoly system” that for nearly four decades allowed a single private nonprofit to be the sole contractor managing the country’s Organ Procurement and Transplantation Network (OPTN), White House press secretary Karine Jean-Pierre told reporters Friday.
“Everybody knows the system has been broken for years, with heartbreaking consequences,” she said. “Now, with the president’s signature, we are taking significant steps to improve it.”
The Securing the U.S. Organ Procurement and Transplantation Network Act stems from rising criticism of the United Network for Organ Sharing (UNOS) and the OPTN from lawmakers on both sides of the aisle. In 2022, the Senate Finance Committee issued a report and convened multiple hearings focusing on the various “failures” of the system that led to long waits and deaths.
Sen. Ron Wyden, D-Oregon, chair of that committee and one of the act’s sponsors, said in a statement. “For too long, thousands of families have had to watch a loved one struggle while waiting for an organ transplant because the system has been inefficient and unaccountable. With this law, that starts to change. There is going to be accountability, know-how, and improvements so more Americans are connected with a life-saving transplant.”
UNOS, the sole nonprofit that has been managing the network, coordinating transplants and procurements and monitoring patient safety, has previously said that it welcomed any plans to reform the national system. The group on Friday said it does “not oppose this legislation” and reiterated its support of “a more competitive and open bidding process” in statements to reporters.
Read more here: https://www.fiercehealthcare.com/providers/biden-signs-bill-introducing-more-competition-us-organ-transplant-network
 Organ Procurement and Transplantation Network Modernization Initiative
Organ Procurement and Transplantation Network Modernization Initiative
Health Resources and Services Administration (HRSA) Administrator Carole Johnson released this statement following Thursday’s (July 27, 2023) passage of the Securing the U.S. Organ Procurement and Transplantation Network Act; the Senate passed the bill Thursday evening.
“The Health Resources and Services Administration shares Congress’ goal of making the Organ Procurement and Transplantation Network (OPTN) work better for the more than 100,000 people on the waiting list for organs. Individuals on the wait list, organ donors, and their families deserve an OPTN governed by an independent, representative board and supported by best-in-class technology, processes, policy, and people. As HRSA announced in March, through our OPTN Modernization Initiative, “We are leaning in and taking
action to make this a reality.”
Learn more about HRSA’s Organ Procurement and Transplantation Network Modernization Initiative.
 Living Donor Protection Act of 2023: HR 2923 – Bill Introduced by Mr. Nadler (for himself, Mr. Balderson, Ms. Blunt Rochester, Mr. Costa, Mr. Curtis, Ms. DeGette, Mrs. Miller-Meeks, Mr. Murphy, and Mr. Bacon)
Living Donor Protection Act of 2023: HR 2923 – Bill Introduced by Mr. Nadler (for himself, Mr. Balderson, Ms. Blunt Rochester, Mr. Costa, Mr. Curtis, Ms. DeGette, Mrs. Miller-Meeks, Mr. Murphy, and Mr. Bacon)
Support legislation that would prohibit discrimination in the pricing or availability of life, disability or long-term care insurance for living donors. The bill also adds living donation to the Family and Medical Leave Act (FMLA).
This bill prohibits certain insurance carriers from discriminating against, and provides other protections for, living organ donors. Insurance carriers may not deny, cancel, or otherwise impose conditions on policies for life insurance, disability insurance, or long-term care insurance based on an individual’s status as a living organ donor.
This bill also codifies organ-donation surgery as a serious health condition that entitles eligible employees to job-protected medical leave (FMLA). Additionally, HHS must also update educational materials on live organ donation to include information about the benefits of live organ donation and about access to insurance for living organ donors.
Learn more here: https://www.congress.gov/bill/118th-congress/house-bill/2923/text/ih?overview=closed&format=xml
The End-Stage Renal Disease (ESRD) Treatment Choices (ETC) Model is intended to encourage greater use of home dialysis and kidney transplants for Medicare beneficiaries with ESRD, while reducing Medicare expenditures and preserving or enhancing the quality of care.
30% of the randomized nephrology clinicians and dialysis facilities have substantial financial implications on the basis of the home dialysis rate— and the transplant rate, which incorporates both transplant wait listing and living donor transplantation for patients on dialysis aged <75 years. Learn more here: https://kidney360.asnjournals.org/content/2/10/1677
Kidney Transplant Conversations features diverse voices and experiences of donating, receiving, and caring for this gift of life. Episodes feature interviews with patients, caregivers, advocates, donors, healthcare providers, and community leaders.
This conversational podcast focuses on quality healthcare delivery, highlighting the innovative approaches kidney transplant recipients and providers are applying to improve care. Learn more here: https://podcasts.apple.com/us/podcast/kidney-transplant-conversations/id1575609931
 NEWSFALSH! August 13, 2021: CDC embraces FDA’s authorization of 3RD vaccine dose for kidney transplant patients!
NEWSFALSH! August 13, 2021: CDC embraces FDA’s authorization of 3RD vaccine dose for kidney transplant patients!  Support HR 7663 and make Telehealth Evergreen. Crozer Connor, Senior Legislative Assistant/Health Policy Lead, Office of Representative Mike Thompson (D-CA), Co-Chair, Congressional Telehealth Caucus is leading the way. As legislators to co-sponsor and pass this bill.
Support HR 7663 and make Telehealth Evergreen. Crozer Connor, Senior Legislative Assistant/Health Policy Lead, Office of Representative Mike Thompson (D-CA), Co-Chair, Congressional Telehealth Caucus is leading the way. As legislators to co-sponsor and pass this bill.
A rapid increase in “virtual” visits during the COVID-19 pandemic could transform the way physicians provide care in the United States going forward, according to a new study led by researchers from NYU Grossman School of Medicine.
As social distancing continues to be a necessity, physician practices have begun to offer their patients much-needed access to virtual care thru TeleMedicine. This offering has increased patient volume and revenue, (which were down 60 percent and 55 percent respectively). Tele-medicine is now viewed as an urgent necessity to replace in-person care in an effort to minimize patient exposure and PPE. It’s been so effective we want it to be forever available and covered by medical insurance.
 COVID 19 is forcing transplant centers to cancel live kidney donor transplants and put “donor testing” on hold. Hopeful recipients are emotionally struggling with this disruptive and unpredictable standstill. Their thought bubbles sound like: “Will my donor change their mind? Will my GFR hold? Will I be forced on dialysis or be taken off the list as I get sicker?”
COVID 19 is forcing transplant centers to cancel live kidney donor transplants and put “donor testing” on hold. Hopeful recipients are emotionally struggling with this disruptive and unpredictable standstill. Their thought bubbles sound like: “Will my donor change their mind? Will my GFR hold? Will I be forced on dialysis or be taken off the list as I get sicker?”
While these concerns are realistic deal-breakers, an intentional shift in perspective could reveal a brighter blessing in disguise. Why is this possible? Because putting life’s plans on hold presents an expanded window of opportunity to refine and strengthen one’s goals.
It’s a matter of choice. You can sit frozen in fear or you can shift what’s beyond your control to work to your advantage. Learn more here
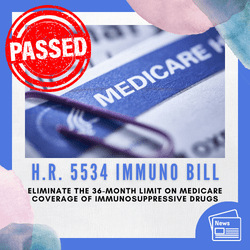 2020 Update! This bill PASSED and will become law January 1, 2023. Comprehensive Immunosuppressive Drug Coverage for Kidney Transplant Patients Act of 2019, H.R. 5534, was introduced by representatives Ron Kind (D-WI) and Representative Michael Burgess (R-TX) on December 23, 2019. The legislative hearing is set for January 8th, 2020—tell congress to vote YES.
2020 Update! This bill PASSED and will become law January 1, 2023. Comprehensive Immunosuppressive Drug Coverage for Kidney Transplant Patients Act of 2019, H.R. 5534, was introduced by representatives Ron Kind (D-WI) and Representative Michael Burgess (R-TX) on December 23, 2019. The legislative hearing is set for January 8th, 2020—tell congress to vote YES.
THE GOAL: Was to eliminate the 36-month limit on Medicare coverage of immunosuppressive drugs for kidney transplant recipients without coverage—and save Medicare 400 million over 10 years.
Be sure to thank your members of congress for passing this bill!
 New Partnership Enhances Kidney Health Awareness, Educates Professionals & Spurs Innovation. Washington, D.C., November 4, 2019. The first major effort by a President to improve kidney health (since Medicare began covering end-stage renal disease patients back in 1972), is taking flight in a new partnership between the National Kidney Foundation (NKF), American Society of Nephrology (ASN) and the U.S. Department of Health and Human Services(HHS). This historic partnership intends to collaborate on public awareness initiatives outlined in the Executive Order on Advancing American Kidney Health. The ultimate goal is to transform kidney health by enhancing public awareness, educating clinical professionals and spurring innovation by entities serving the kidney community. Click to learn more
New Partnership Enhances Kidney Health Awareness, Educates Professionals & Spurs Innovation. Washington, D.C., November 4, 2019. The first major effort by a President to improve kidney health (since Medicare began covering end-stage renal disease patients back in 1972), is taking flight in a new partnership between the National Kidney Foundation (NKF), American Society of Nephrology (ASN) and the U.S. Department of Health and Human Services(HHS). This historic partnership intends to collaborate on public awareness initiatives outlined in the Executive Order on Advancing American Kidney Health. The ultimate goal is to transform kidney health by enhancing public awareness, educating clinical professionals and spurring innovation by entities serving the kidney community. Click to learn more
 President Orders Kidney Care Revamp, CMS Proposes Payment Models. On July 10, 2019, President Trump signed “Executive Order on Advancing American Kidney Health,” reforming care for kidney disease. The goals of the executive order are to provide care before dialysis is needed and to increase transplants and home dialysis.
President Orders Kidney Care Revamp, CMS Proposes Payment Models. On July 10, 2019, President Trump signed “Executive Order on Advancing American Kidney Health,” reforming care for kidney disease. The goals of the executive order are to provide care before dialysis is needed and to increase transplants and home dialysis.
While the president was signing the executive order, the Centers for Medicare & Medicaid (CMS) rolled out five proposed payment models for kidney care.
 Reps. Jerrold Nadler, D-NY, and Jaime Herrera Beutler, R-Wash.,joined Sens. Kirsten Gillibrand, D-NY, and Tom Cotton, R-Ark., on Feb. 14 to introduce the Living Donor Protection Act of 2019. According to a press release issued by Nadler’s office, the Department of Labor issued a legal opinion last year stating that individuals who choose to donate an organ are covered under the Family Medical Leave Act (FMLA). The Living Donor Protection Act would codify the Department of Labor’s guidance to protect living donors in the private and civil service sector, removing one of the largest barriers to organ donation, they said.
Reps. Jerrold Nadler, D-NY, and Jaime Herrera Beutler, R-Wash.,joined Sens. Kirsten Gillibrand, D-NY, and Tom Cotton, R-Ark., on Feb. 14 to introduce the Living Donor Protection Act of 2019. According to a press release issued by Nadler’s office, the Department of Labor issued a legal opinion last year stating that individuals who choose to donate an organ are covered under the Family Medical Leave Act (FMLA). The Living Donor Protection Act would codify the Department of Labor’s guidance to protect living donors in the private and civil service sector, removing one of the largest barriers to organ donation, they said.
Ensuring that donors have the time they need to heal following donation and can continue to afford insurance going forward will give donors more certainty and encourage more Americans to make the gift of living organ donation.” The legislators cited a 2014 study in the American Journal of Transplantation that indicated that as many as 27% of living organ donors have trouble securing or paying for insurance after their procedures because of discriminatory practices.
The Living Donor Protection Act would protect living organ donors and promote organ donation in three ways, according to the press release: (1) the act prohibits life, disability and long-term care insurance companies from denying or limiting coverage and from charging higher premiums for living organ donors; (2) it amends the Family and Medical Leave Act of 1993 to specifically include living organ donation as a serious health condition for private and civil service employees; and, (3) the act directs HHS to update their materials on live organ donation to reflect these new protections and encourage more individuals to consider donating an organ.

Dec 19, 2018, Envarsus XR (tacrolimus extended-release tablets) to prevent organ rejection in de novo kidney transplant. FDA approved Envarsus XR for the prophylaxis of organ rejection in kidney transplant patients converted from tacrolimus immediate-release formulations in 2015 which has been used in the conversion setting in more than 90% of the transplant centers in the U.S.
The approval for “de novo” use (from the beginning of a patients transplant journey) provides an important new treatment option for kidney transplant patients and providers, where significant unmet need currently exists.
Envarsus XR technology uses a MeltDose drug delivery technology to enhance the oral bioavailability and control the release of a drug, especially low water-soluble or insoluble drugs. The goal of this technology is to improve efficacy and/or reduce side effects. Particle size plays a vital role in bioavailability. Unlike conventional and nanocrystal drug delivery formulations which use larger particles that are more difficult to absorb, MeltDose technology enhances bioavailabilty by reducing the drug to the smallest-possible particle size-down to single molecules. The smaller particle size enables better dissolution and absorption.
Click here to learn more about ENVARSUS XR including indication and Important Safety Information for ENVARSUS XR (tacrolimus extended-release tablets).
Non-Invasive Kidney Transplant Surveillance test . AlloSure (by CareDX) is a non-invasive blood test that assesses organ health. The test can determine active rejection in kidney transplant patients.
AlloSure measures donor-derived cell-free DNA (dd-cfDNA) in the blood, which is an indicator of kidney injury and sign of rejection.